Understanding the Calibration Process for Analytical Balances
Understanding the Calibration Process for Analytical Balances
Blog Article
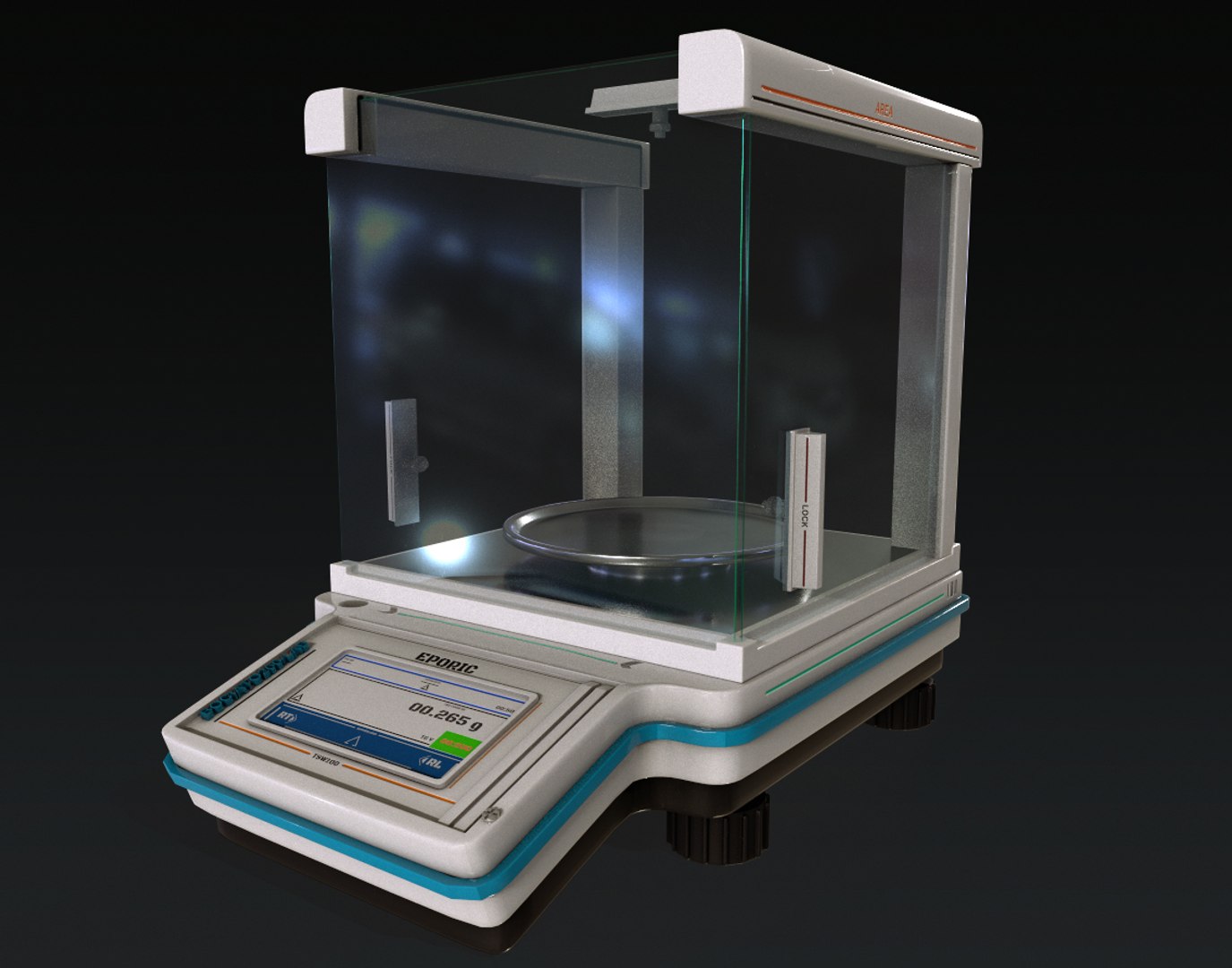
In the realm of high-precision laboratory work, analytical balances play a pivotal role in ensuring accuracy and reliability in measurements. These instruments are essential for various scientific applications, from preparing chemical solutions to conducting research in pharmaceutical developments. Understanding the calibration process for these balances is crucial, as it directly influences the integrity of the results obtained in laboratory settings.
Calibration serves as the backbone of maintaining the accuracy and precision of analytical balances. It involves adjusting the balance to align with standard measurements, thereby eliminating systematic errors that could compromise experimental outcomes. Manufacturers like W&J Instrument specialize in providing high-quality electronic balances and laboratory instruments, emphasizing the importance of proper calibration in achieving consistent and dependable results. This article will explore the calibration process, highlighting its significance, methodology, and best practices for ensuring optimal performance of analytical balances in any laboratory environment.
Importance of Calibration
Calibration is essential for maintaining the accuracy and reliability of high-precision laboratory analytical balances. These instruments play a crucial role in various scientific research and development processes, where even the slightest measurement deviation can lead to significant errors in experimentation and results. Regular calibration ensures that the balance provides precise measurements, which is fundamental for obtaining valid and reproducible data.
In laboratories, the need for consistency cannot be overstated. For researchers and scientists relying on analytical balances, calibration is not just a routine task but a critical step in quality control. It helps identify any discrepancies that may arise from environmental factors or mechanical wear, thus safeguarding the integrity of the measurements. When analytical balances are correctly calibrated, researchers can have greater confidence in their findings, which can impact the credibility of their work.
Moreover, compliance with industry standards and regulations is often tied to the calibration process. Many laboratory protocols require that instruments be regularly calibrated to ensure adherence to established norms. By investing in proper calibration practices, laboratories not only enhance their operational efficiency but also protect themselves from potential liabilities that can arise from incorrect measurements. In this way, calibration emerges as a key component in upholding the standards of scientific rigor in laboratory environments.
Calibration Techniques
Calibration of analytical balances is essential for ensuring accurate measurements in laboratory settings. One of the most common techniques involves the use of certified calibration weights. These weights are known for their accuracy and come in various denominations, allowing for a range of calibration options. During the calibration process, the balance is usually adjusted to display the correct weight corresponding to the standard weights used. This process should be performed at regular intervals and whenever the balance is moved or after any significant changes in environmental conditions.
Another crucial calibration technique is the multi-point calibration method. This involves testing the analytical balance at several points across its operational range, typically covering both low and high weight capacities. By using multiple standard weights, technicians can create a calibration curve that represents the balance's performance. This helps identify any non-linearities or deviations in the readings and allows for more precise adjustments. Multi-point calibration is particularly beneficial for balances used in critical applications where accuracy is paramount.
Environmental factors also play a significant role in the calibration process. Factors such as temperature, humidity, and vibrations can affect the balance's performance. Therefore, many laboratories control these conditions to maintain consistent calibration standards. Additionally, performing calibration in a draft-free, stable environment helps ensure that the analytical balance functions accurately. W&J Instrument emphasizes the importance of regular calibration and proper environmental controls to optimize the performance of their high-precision laboratory analytical balances.
Common Calibration Errors
One of the most frequent calibration errors occurs when the balance is not leveled properly. An unlevel balance can cause significant discrepancies in readings, leading to inaccurate measurements. It is crucial to ensure that the analytical balance is positioned on a stable, flat surface and that the leveling feet are adjusted correctly. Routine checks for leveling should be a standard part of the calibration process to minimize this common issue.
Another common error is the use of calibration weights that are not within the specified tolerance range of the balance. Using weights that do not match the capacity and resolution of the balance can result in erroneous calibration settings. To achieve optimal accuracy, it is essential to utilize certified calibration weights that conform to the industry's standards, ensuring that all measurements are precise and reliable.
High Precision Balance Manufacturers
Environmental factors can also contribute to calibration errors. Fluctuations in temperature, humidity, and air currents can affect balance performance, altering the measurements taken. Properly controlling the environment where the analytical balance operates is vital. Utilizing draft shields and maintaining a consistent climate in the laboratory can help mitigate these influences, ensuring consistent and accurate calibration results.
Maintaining Calibration Standards
Maintaining calibration standards is essential for ensuring the accuracy and precision of high-precision laboratory analytical balances. Regular calibration against known standards minimizes errors and enhances the reliability of measurements. It is crucial to establish a routine calibration schedule based on the usage frequency and environmental conditions of the laboratory. Operators should document all calibration activities to maintain a detailed history, which can help in identifying potential issues and trends over time.
In addition to routine calibrations, it is vital to ensure that the calibration weights used are themselves traceable to national or international standards. This traceability guarantees that the values used for calibration are accurate and reliable. Using calibrated weights that are appropriate for the capacity of the balance being calibrated is also important for maintaining measurement integrity. Regularly verifying the calibration weights and storing them in a controlled environment will help prevent any deterioration in accuracy.
Furthermore, it is beneficial to train laboratory personnel in proper handling techniques and maintenance of the analytical balances. Understanding the fundamental principles of calibration and the factors that can influence balance performance, such as temperature changes and vibrations, will equip staff to take the necessary precautions. By fostering a culture of quality and accountability in calibration processes, laboratories can significantly improve their results, ensuring compliance with industry standards and enhancing overall operational efficiency.
Report this page